FRAGEN & ANTWORTEN
How are new products created?
In most cases, the basic raw material is bisphenol A diglycidyl ether. With this as the basis, an almost infinite variation of formulations is possible depending on the addition of other raw materials. This results in products that are, for example, accelerated, rheologically adapted, viscoplasticized, thermally conductive or electrically conductive. We develop epoxy resin formulations adapted to the respective requirements, from which optimum products are created after curing.
What are epoxy resins able to do?
Epoxy resins are thermosetting plastics which, in contrast to thermoplastics, are irreversibly cross-linked after curing, i.e. they do not become liquid again at higher temperatures. The typical properties of epoxy resins are:
- Good chemical resistance
- Hard (or flexible in our case)
- Good mechanical properties
- Good temperature resistance
- Good adhesion
- Good moisture resistance
- Good electrical insulation properties
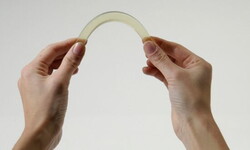
Epoxy resins - as flexible as silicone. Is that possible?
EPOXONIC - specialist for flexible resin systems.
Moulding materials made of epoxy resin are normally known to be particularly hard compared to other plastics. However, we also develop flexible epoxy resins. Flexible here means that we can produce molded materials with Shore hardness < A50 and a glass transition temperature well below ‑20°C. The well-known advantages of epoxy resins, is that they are particularly resistant to chemicals and/or temperature. The curing mechanism here requires hot curing, which is initiated either catalytically or by anhydrides. An example of a flexible, catalytic system is EPOXONIC 376
How are epoxy resins actually cured?
Amine curing
One way of curing epoxy resins is to react with amines.

There is a wide range of amines that can be used for curing. Whether aliphatic, cycloaliphatic, aromatic, long-chain or short-chain, each of these amines can be reacted with epoxy resin. Depending on the application and hardener used, various properties are possible: room temperature curing or hot curing, glass transition temperature in the range of 50 - 150°C, 2-component systems (or 1-component systems).
A good example of this is our EPOXONIC 382.
Anhydride curing
One way of curing epoxy resins is to react with anhydrides.
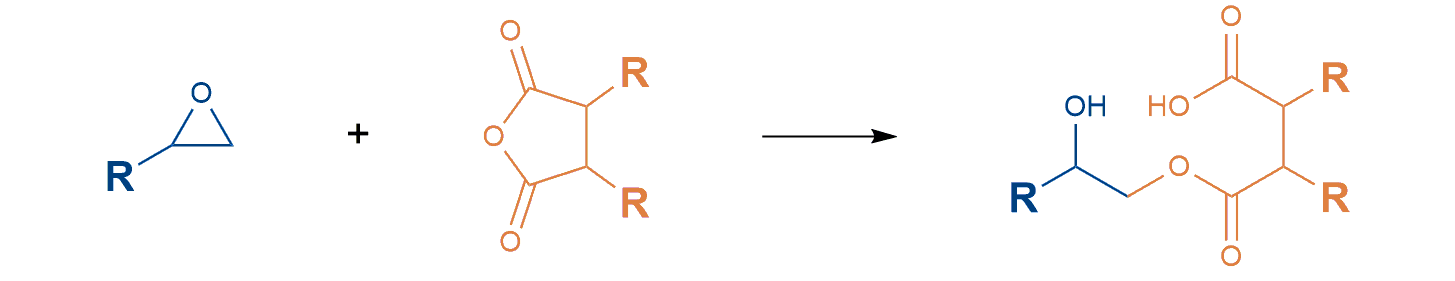
In anhydride curing, the anhydride is opened in the first step and reacts with the diglycidyl ether in the second step. Curing temperatures above 100°C are required for this step-by-step reaction. In return, this system allows a processing time of up to several weeks. Further advantages of this system are low reaction shrinkage, the possibility of casting large volumes without loss of reaction control and good processability. This hardener system allows one- or two-component formulations.
Catalytic curing
One way of curing epoxy resins is polymerization using catalysts
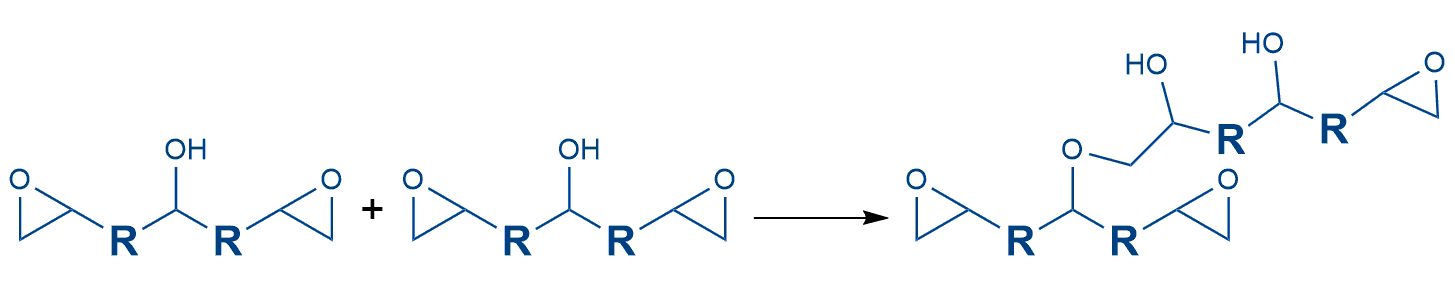
Like anhydride curing, catalytic curing takes place at temperatures above 100°C. Here, hydroxyl groups present in the diglycidyl ether can react with the epoxy ring of another molecule. Tertiary amines or imidazoles, for example, can be used as catalysts. These systems are characterized by a long use duration and very high glass transition temperatures of up to 180°C can be achieved. These formulations are possible in both one- and two-component formulations.
A good example of this is our EPOXONIC 361